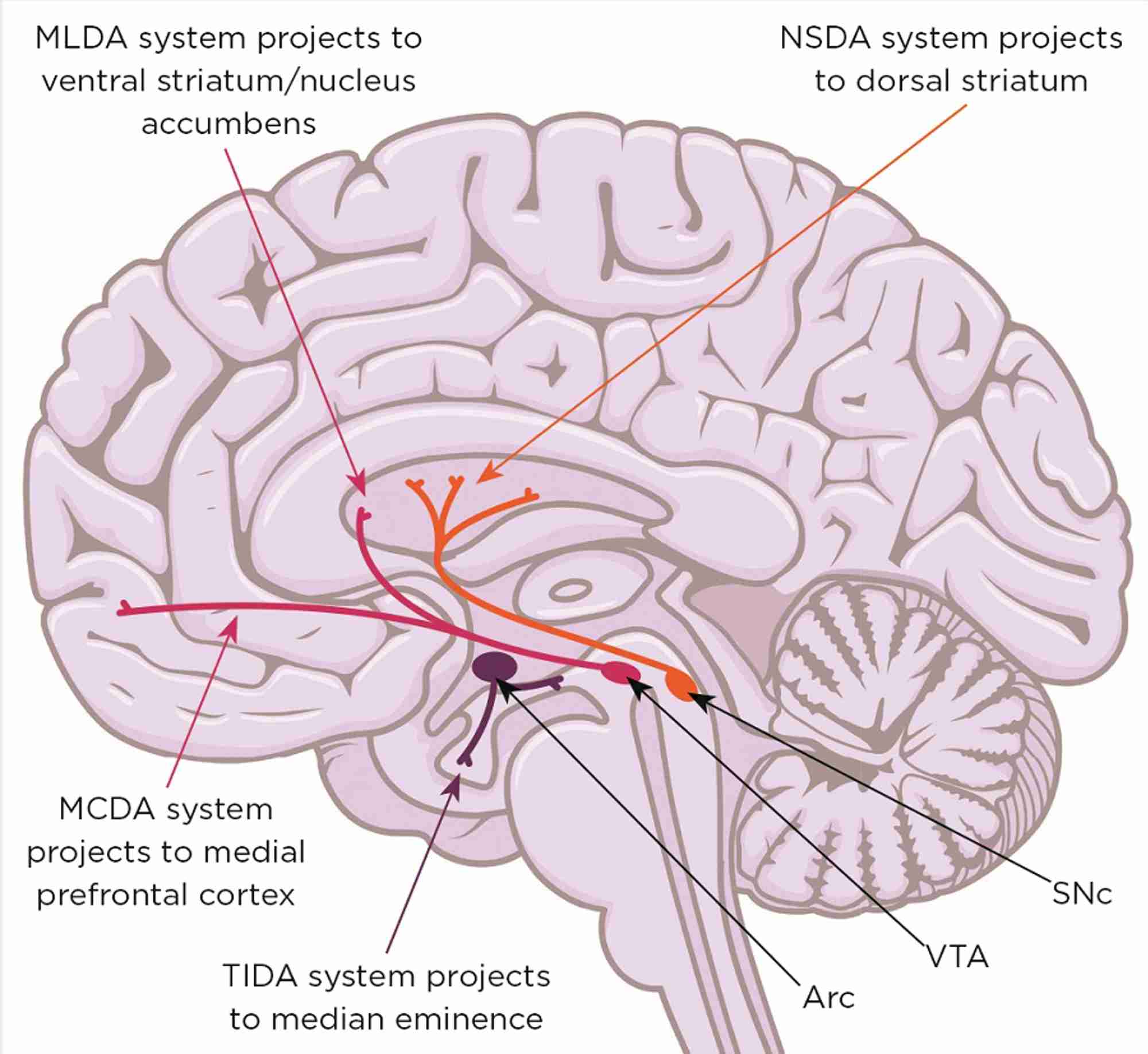
Figure 1. Midbrain dopaminergic systems. Neurones in the substantia nigra pars compacta (SNc) form the nigrostriatal dopaminergic (NSDA) system (involved in sensorimotor regulation) and degenerate in the movement disorder Parkinson’s disease. Neurones in the ventral tegmental area (VTA) form the mesolimbic dopaminergic (MLDA) system (involved in reward and feeding) and the mesocortical dopaminergic (MCDA) system (involved in decision making and working memory); their dysfunction is involved in schizophrenia, attention deficit/hyperactivity disorder, autism spectrum disorders, depression and addiction. Arc, arcuate nucleus; TIDA, tuberofundibular dopaminergic system. Brain image: Shutterstock
Sex and stress hormones play a critical role in mammalian brain development by regulating the number, location and connectivity of neurones via potent influences on neurogenesis, programmed cell death, neuronal migration and synaptogenesis.
Exposure to testosterone, oestradiol and glucocorticoid hormones (GCs) has to be critically timed and co-ordinated for correct orchestration of brain organisation and correct control of two key processes that are fundamental for survival of the species. These are reproduction and the ability to cope with stress, both of which are differentially programmed in males and females.
As (neuro)endocrinologists, we know that male and female brains develop in different hormonal ‘soups’ during a critical developmental window for sexual differentiation of the brain.1 This is largely due to a surge in testosterone production by the male testes at a time when ovarian steroidogenesis in females is relatively quiescent. This occurs during late gestation and the early postnatal period in rats and mice, and probably during mid-gestation in humans.
Conversion of the testosterone surge to oestradiol by aromatase enzymes in the brain allows the male (but not the female) brain to be exposed to oestradiol. This is a key factor in masculinising/defeminising the circuitry of the hypothalamo-pituitary-gonadal (HPG) axis, which suppresses the ability of the male hypothalamus to respond to oestradiol in adulthood. In contrast, retention of oestradiol sensitivity by the female hypothalamus is necessary for ovulation and reproductive behaviours.
REDRESSING AN IMBALANCE
Neuroscientists studying brain regions other than the hypothalamus have largely ignored brain sex differences, choosing to work with the male of the species to avoid the ‘inconvenience’ of hormonal fluctuations.
'We must address the fact that 80% of single sex studies use males, 4% compare both sexes, and some fail to identify sex. It is because of this that most of what we know about the brain actually refers to the male brain'
For the population at large, a denial of biological brain sex differences in favour of sociocultural stereotyping may be understandable: equality of the sexes is a hard-fought battle and the concept that the brains of men and women are inherently different, with the implication that one (usually the male) is superior to the other, has been challenged as being politically incorrect and even retrogressive.
However, a decade ago, a review with the wonderful title ‘Why sex matters for neuroscience’ was, essentially, a wake-up call.2 It highlighted mounting evidence for biological sex differences in the structure and function of higher brain centres in humans and animals, which influence learning, memory and emotion (hippocampus, amygdala, prefrontal cortex).
Moreover, many of these differences appear to be programmed by the early sex hormone environment and are thought to underpin sex differences that abound in the susceptibility to and nature of virtually all brain disorders. This highlights an urgent need to understand brain sex differences if men and women are to be treated most effectively when brain functioning goes wrong. Therefore, we must address the fact that 80% of single sex studies use males, 4% compare both sexes, and some fail to identify sex. It is because of this that most of what we know about the brain actually refers to the male brain.
THE HPA AXIS
Although not as obvious as the HPG axis, the response of the hypothalamo-pituitary-adrenal (HPA) axis to stress is also sexually differentiated and is often characterised as ‘fright, fight or flight’ in males, but ‘tend, defend or befriend’ in females. Sex differences in the developmental sex hormone environment undoubtedly play a role here, as does exposure to GCs, the end products of the HPA axis.
Towards the end of gestation in most mammalian species, both sexes experience a critically timed rise in the bioavailability of GCs released from the fetal/maternal adrenal cortex.3 This is important for normal maturation of many tissues and organs, including the lungs (hence the value of giving GCs to mothers when premature delivery is threatened) and the brain. Thus, in males (not females), there may be competing epigenetic influences, as GCs briefly overlap with testosterone or its oestrogenic metabolites on processes that sculpt the developing brain, thereby contributing to normal sexual dimorphisms.
'Endocrinology can clearly make important contributions to understanding the origins of brain disorders and their sex bias'
However, if GC levels are inappropriately elevated, either by intrauterine/ neonatal exposure to stressors or excessive therapeutic use in perinatal medicine (e.g. repeated courses of GCs may be given when threatened premature birth is delayed, which happens in about 50% of cases), brain structure, function and behaviour may be adversely affected in later life, a phenomenon often termed GC-programming. For example, GCs can interfere with normal brain masculinisation, leading to feminisation of reproductive behaviours, and can also impair HPA axis reactivity to stress and, therefore, physiological stress-coping mechanisms.
IMPACT ON MENTAL HEALTH
As failure of stress-coping mechanisms is an established risk factor for developing brain disorders, and early-life adversity is linked with mental health problems in later life, many studies have focused on GC programming of HPA axis failure as an underlying mechanism.4
However, the mid-brain dopaminergic systems (mDAs; Figure 1) also play a critical role in the behavioural responses to stress, enabling the storage and recall of appropriate coping behaviours. Not surprisingly, therefore, malfunction of dopamine-dependent stress-coping circuitry is a prevalent factor in many neurological and neuropsychiatric conditions. Furthermore, most of these conditions have a non-genetic, developmental component associated with perinatal stress exposure, as well as a sex bias.5,6
For example, conditions involving mDAs that affect males more than females include schizophrenia, attention deficit/hyperactivity disorder, autism spectrum disorders and Parkinson’s disease, and are associated with stressors such as obstetric complications, exposure of the pregnant mother to natural disasters, famine, death of a loved one, low socioeconomic status and infections. Anxiety and depression are more prevalent in females and often associated with childhood abuse.
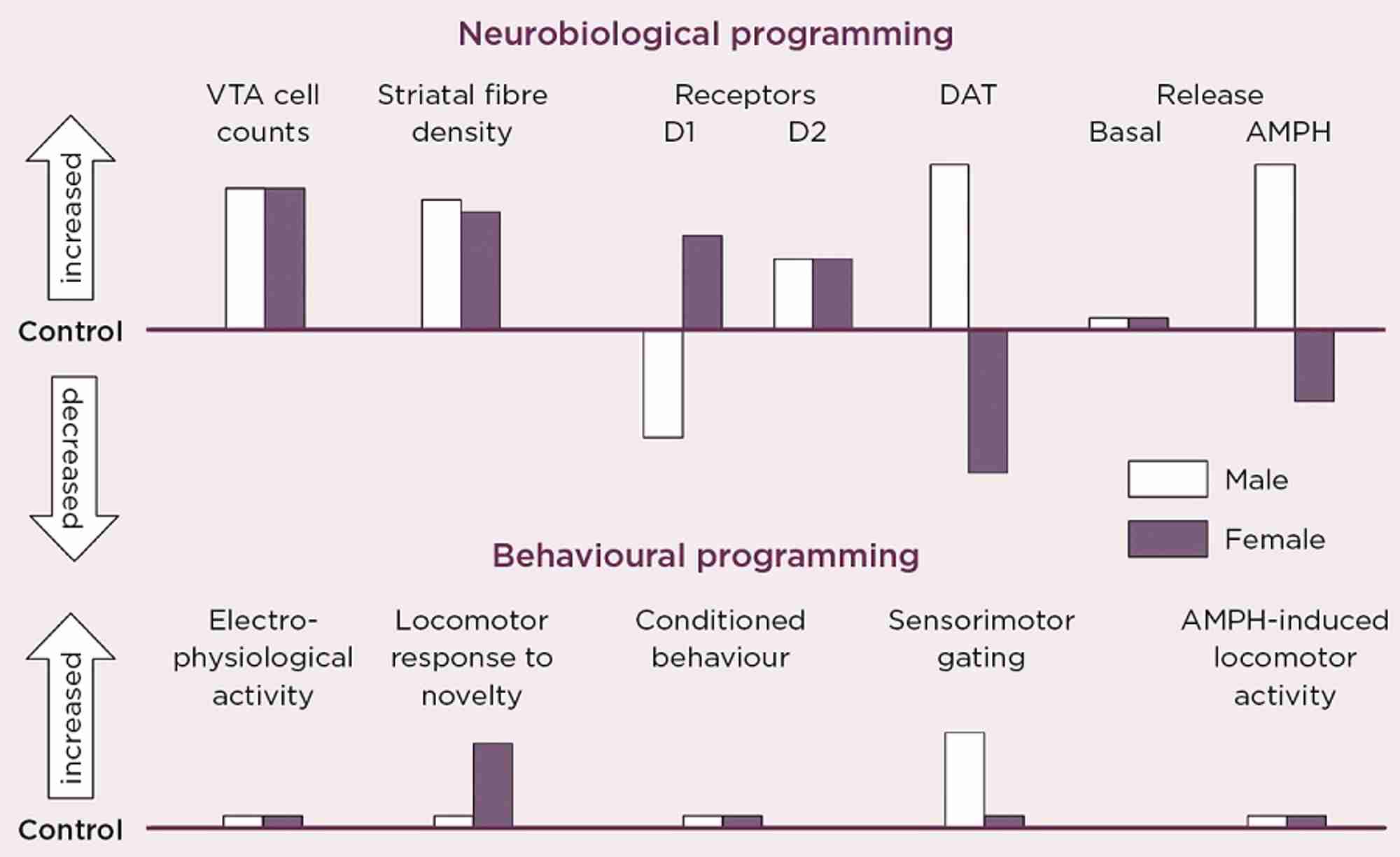
Figure 2. Schematic summary of the directional change in neurobiological and behavioural indicators of VTA dopaminergic activity in adult male and female rats after antenatal glucocorticoid treatment (AGT). Male and female rats exposed to AGT (dexamethasone, 0.5 µg/ml, in drinking water on gestational days 16-19) and controls (dams received normal drinking water) were tested in adulthood. Neurobiological programming (A): The numbers of dopaminergic neurone cell bodies in the VTA and the density of their terminal fibres in the nucleus accumbens (NAc) core and shell regions of the striatum were assessed after immunostaining for tyrosine hydroxylase. Expression levels of dopaminergic receptors (D1, D2) and the dopamine transporter (DAT) in the NAc core and shell were assessed using autoradiography. Basal and amphetamine-stimulated DA efflux was measured using in vivo microdialysis. Behavioral programming (B): Electrophysiological measurements in vivo included extracellular recordings from individual putative DA neurons in the VTA of adult male rats and assessments of spike width, firing rate, inter-spike intervals and percentage of action potentials in spike bursts. Tests of conditioned behavior involved Pavlovian learning in response to appetitive cues predictive of food (autoshaping) and cocaine self-administration using a fixed schedule of reinforcement with ascending doses of cocaine. Sensorimotor gating was tested by analyzing efficacy of a weak sensory stimulus to inhibit a reflexive motor response to a subsequent intense sensory event (pre-pulse inhibition of acoustic startle). Locomotor activity induced by intra-peritoneal injections of amphetamine (0, 0.2, 0.4, 0.8 and 1.2 mg/Kg) was recorded using photocell chambers. For full details see Virdee et al 2014 Neuropsychopharmacology 39 339-350.
Our own studies, in rats and mice, exposing fetuses to the synthetic GC, dexamethasone, provide direct evidence that the mDAs are targets for sex-specific GC programming (Figure 2).3 This profoundly alters the numbers and distribution of dopaminergic neurones and astrocytes in the mid-brain regions of the adult brain. It was accompanied by robust changes in molecular markers of dopamine transmission, but the direction of change was diametrically opposite in males and females for the D1 receptor, the dopamine transporter and dopamine release in response to amphetamine, all of which would predict great effects on behaviours controlled by this circuitry.
However, this was not the case. Certain behaviours were not affected in either sex, whereas small effects were seen in some behaviours only in males, and in others only in females. We conclude that these changes represent enduring adaptive mechanisms, which secure protection against early environmental adversity. However, there are striking differences by which males and females achieve apparent behavioural resilience, and sometimes these mechanisms fail in a sex-specific manner. These findings show that, although the developing brain can clearly adapt to environmental challenges, it does so at the cost of operating outside normality (allostasis), thereby predisposing an individual to malfunction of stress-coping processes, according to whether you are male or female.
A ROLE FOR ENDOCRINOLOGISTS
Mental health issues are at the top of the agenda, not just for biomedical science, but for governments and global organisations (such as the World Health Organization, the United Nations and charities). There is currently a focus on removing the stigma of discrimination attached to mental disorders, as well as the reluctance of those who suffer from these conditions to come forward for diagnosis and treatment.
Endocrinology can clearly make important contributions to understanding the origins of brain disorders and their sex bias. This is crucial for realising the potential to reverse or prevent these debilitating conditions and for recognising that approaches need to be differentially tailored to the needs of men and women.
Glenda Gillies
Emeritus Professor in Neuroendocrine Pharmacology, Imperial College London, Hammersmith Hospital
REFERENCES
1. Gillies GE & McArthur S 2010 Pharmacological Reviews 62 155–198.
2. Cahill L 2006 Nature Reviews Neuroscience 7 477–484.
3. McArthur S et al. 2016 Brain Structure & Function 221 2459–2475.
4. Reynolds RM 2013 Psychoneuroendocrinology 38 1–11.
5. Gillies GE et al. 2014 Neuroscience 282 69–85.
6. Gillies GE et al. 2014 Frontiers in Neuroendocrinology 35 370–384.








