Functional hypothalamic amenorrhoea (FHA) is the second most common cause of amenorrhoea among premenopausal women.1 Usually associated with negative energy balance, FHA occurs in up to 80% of women with anorexia nervosa, in up to 66% of women who exercise intensely, and it can also be triggered by psychological stress.1 Ultimately, these factors suppress the hypothalamo–pituitary–gonadal axis, resulting in menstrual interruption.
The endocrine imbalance in FHA (largely an adaptive response to conserve energy, Figure 1) disrupts bone health, leading to an increased fracture risk.1 Indeed, in amenorrhoeic women with anorexia nervosa and in oligo-amenorrhoeic athletes, bone microarchitecture and bone mineral density (BMD) are dramatically impaired, resulting in a fracture prevalence almost double that of healthy eumenorrhoeic women.2 Early bone health assessment is therefore essential in these women, especially if amenorrhoea persists for 6 months or more.1
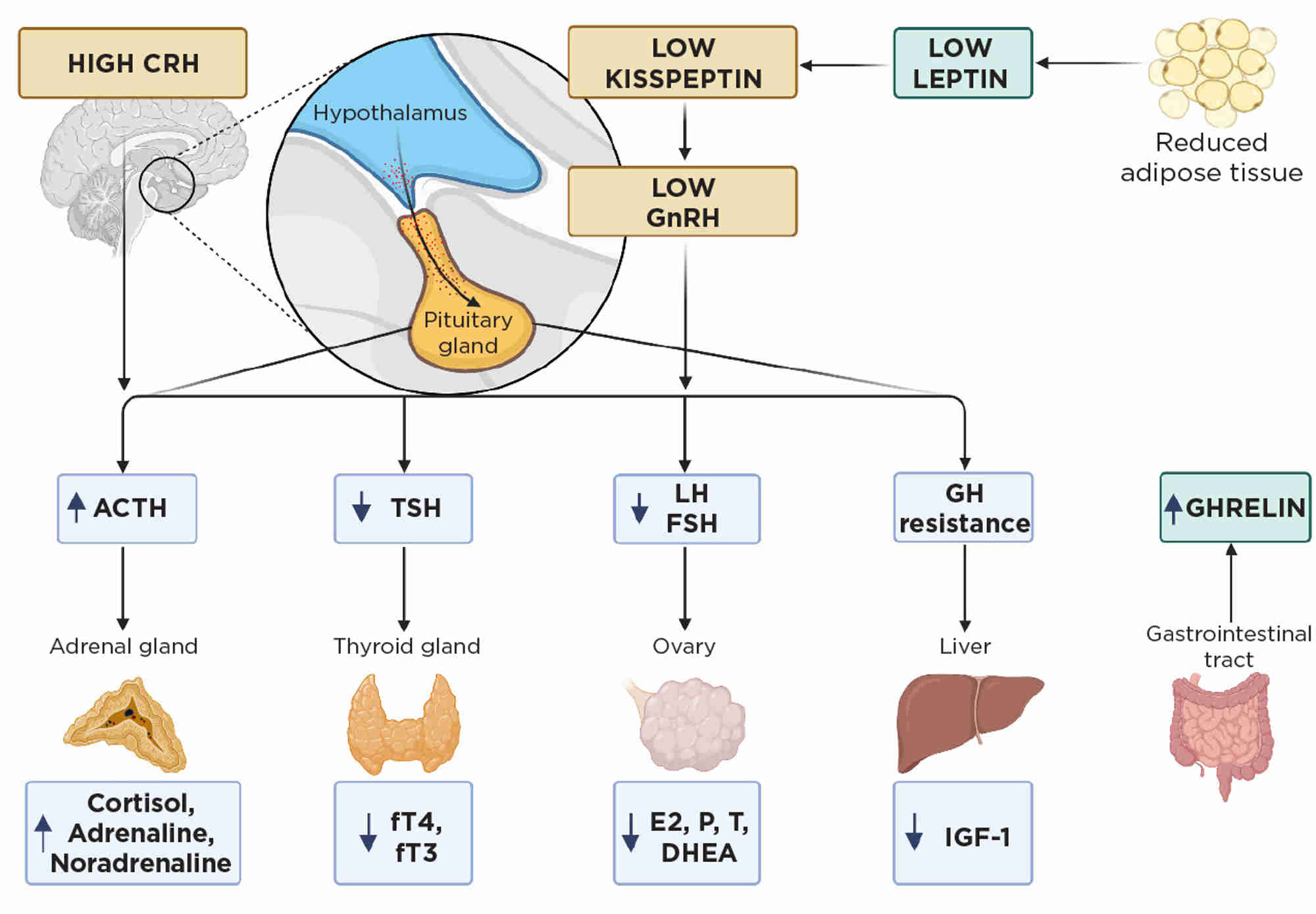
Figure 1. Endocrine imbalance in FHA usually occurs secondary to calorie restriction with low weight in anorexia, excess exercise or high stress. CRH, corticotrophin-releasing hormone; DHEA; dehydroepiandrosterone; E2, oestradiol; FSH, follicle-stimulating hormone; fT3, free tri-iodothyronine; fT4, free thyroxine; GH, growth hormone; GnRH, gonadotrophin-releasing hormone; LH, luteinising hormone; P, progesterone; T, testosterone; TSH, thyrotrophin. Created with BioRender
ENDOCRINE IMBALANCE IN FHA IMPACTS BONE
Bone health in FHA is disrupted primarily due to oestrogen deficiency, causing heightened osteoclastic bone resorption and reduced osteoblastic bone formation through several signalling pathways (Figure 2).
However, additional endocrine changes also negatively impact bone (Figures 1 and 2).3 For example, chronic starvation causes growth hormone resistance, reducing circulating insulin-like growth factor-1 (IGF-1), an osteoanabolic hormone, by up to 50%. In addition, chronic starvation decreases thyrotrophin and thyroid hormones, correlating with lower BMD. Low adiposity in FHA results in reduced leptin, resulting in direct negative effects on bone microarchitecture, and reductions in other osteoanabolic hormones. Furthermore, stress increases cortisol levels which correlate negatively with BMD.3
CLINICAL CONSIDERATIONS
In the endocrine–bone clinic, clinicians should explore factors such as eating patterns, exercise routine, stress and the patient’s understanding of the link between menstrual cyclicity and bone health. The age of onset of amenorrhoea is important, with a lower BMD observed when onset occurs during adolescence compared with adulthood, despite comparable duration of amenorrhoea.3 This highlights the importance of early identification of FHA for bone health.
Women should ensure a calcium intake >700mg/day with serum 25(OH)vitamin D levels >50nmol/l (with supplements if required).1 Lifestyle adjustments focusing on weight gain, nutritional improvements, exercise adjustments and stress reduction to promote menstrual restoration are crucial. Indeed, both weight gain and menstrual resumption independently improve BMD. However, the impact of these measures on BMD improvement varies, with full restoration not guaranteed.1 When amenorrhoea persists despite 6–12 months of efforts to address the aetiology, oestrogen therapy should be considered for bone health.1
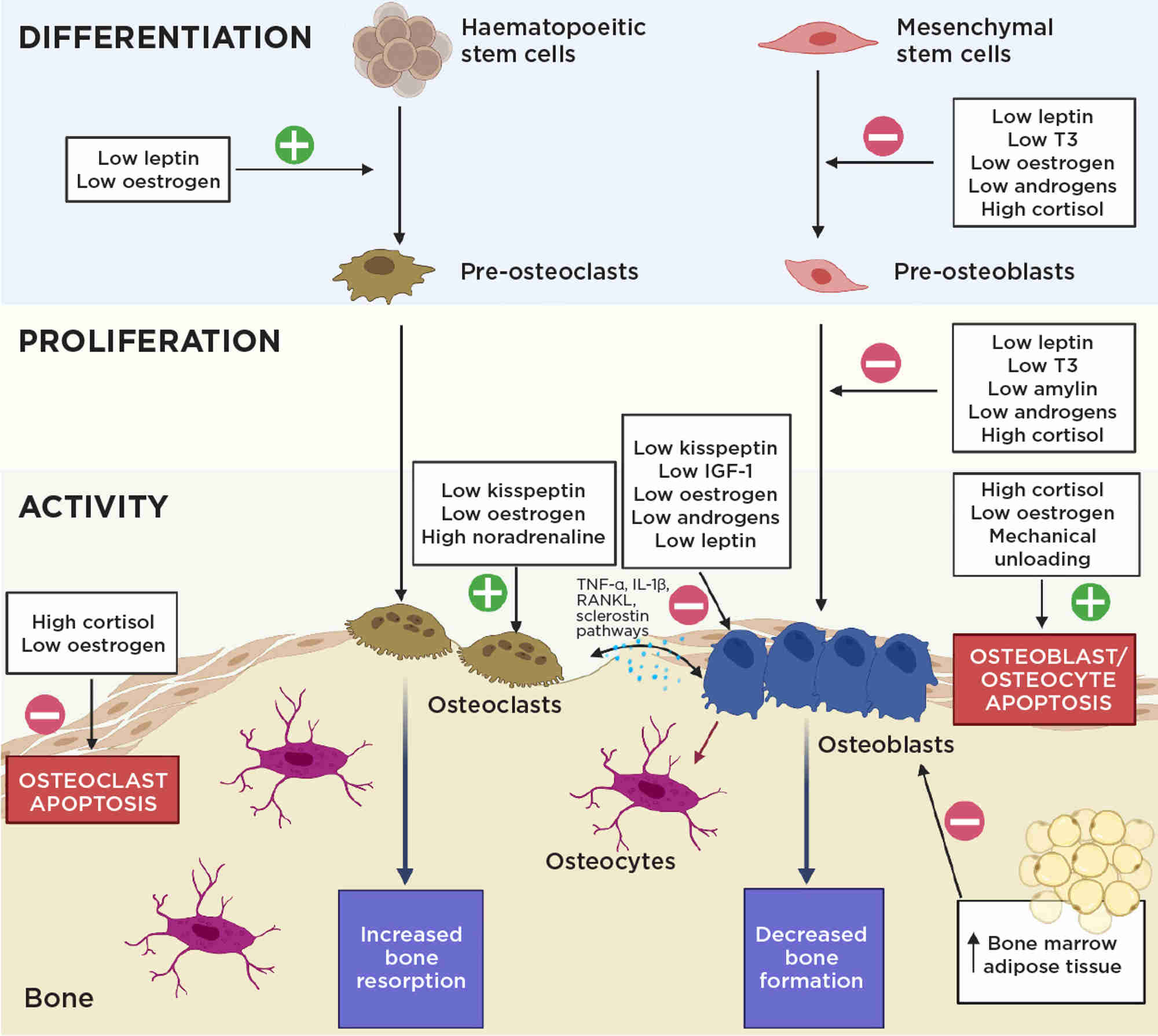
Figure 2. The endocrine imbalance in FHA negatively affects bone homeostasis through a variety of mechanisms. IGF-1, insulin-like growth factor-1; IL-1β, interleukin-1β; T3, tri-iodothyronine; TNF-α, tumour necrosis factor-α. Created with BioRenderFigure 2. The endocrine imbalance in FHA negatively affects bone homeostasis through a variety of mechanisms. IGF-1, insulin-like growth factor-1; IL-1β, interleukin-1β; T3, tri-iodothyronine; TNF-α, tumour necrosis factor-α. Created with BioRender
OESTROGREN REPLACEMENT FOR BONE HEALTH
Current evidence supports the use of transdermal hormone replacement therapy (HRT) (containing ‘physiological’ 17β-oestradiol with cyclic progestin), in preference to oral HRT or combined oral contraceptives (COCP), as the optimal management for bone health in women with FHA.3
This is largely based on a 12-month randomised study involving 121 young oligo-amenorrhoeic athletes, demonstrating that transdermal HRT (containing 100µg 17β-oestradiol twice-weekly) improved lumbar spine and femoral neck BMD by 2–4% more than COCP (containing 30µg ethinyl oestradiol; EE) or no oestrogen treatment. In fact, women receiving COCP exhibited a decline in total hip BMD.4 Importantly, there were no differences in weight or menstrual function during the study, thereby directly attributing the observed bone effects to the properties of transdermal HRT compared with COCP. Consistent with this, a randomised placebo-controlled study showed that supraphysiological EE (35µg) in COCP does not improve BMD in subjects with anorexia nervosa.5
The benefits of transdermal HRT have been further demonstrated over 18 months in women with anorexia nervosa, showing up to 3% improved lumbar BMD.6 Such observations were corroborated by a meta-analysis of 770 women with FHA. Among COCP, conjugated oral oestrogen and transdermal HRT, only use of transdermal HRT was associated with increases in lumbar BMD.7
Collectively, these findings are primarily attributed to the fact that oral (but not transdermal) oestrogen undergoes hepatic first-pass metabolism. This leads to downregulation of the osteoanabolic hormone IGF-1 by up to 30%, thus exacerbating the IGF-1-deficient state existing in FHA, and further compromising bone health.8
In addition, most COCPs utilise supraphysiological doses of EE, hence it remains unclear whether lower oral EE doses or other oral oestrogen forms might be less detrimental, or even beneficial, to bone health. To this end, postmenopausal women on 30µg EE were found to have significantly greater IGF-1 reductions (37%) compared with those receiving 20µg EE (30%), suggesting a dose–effect on IGF-1.9
Furthermore, oral (but not transdermal) oestrogens stimulate sex hormone-binding globulin, which reduces bioavailable oestrogen. Finally, studies to date have employed the standard regimen of COCP, comprising 7 days without oestrogen per cycle, thus interrupting oestrogen exposure. By contrast, transdermal HRT delivers continuous 17β-oestradiol, hence providing sustained oestrogen exposure.
In summary, the benefits of transdermal HRT over COCP probably relate to a combination of lesser IGF-1 suppression, and better oestrogen bioavailability and continuity. However, further, larger randomised studies are warranted to clarify the optimal route, dose and form of oestrogen therapy for bone health.
OTHER AVAILABLE THERAPEUTIC OPTIONS
Bisphosphonates and denosumab can improve BMD in women with FHA, but are not routinely recommended in women with child-bearing potential, given their prolonged half-life and ability to cross the placenta, with potential pregnancy complications.3 Teriparatide can also improve BMD in FHA but its limited course (currently 2 years), need for self-injections and cost all limit its routine use.3 Data are awaited on the newest agent, romosozumab.
EMERGING THERAPEUTICS
Subcutaneous leptin showed promising improvements in markers of bone formation and BMD in women with FHA, yet associated weight loss restricted its appropriateness in FHA. Recombinant IGF-1, when given together with COCP to women with FHA, improves lumbar BMD but has not been developed further, while studies using androgens do not show consistent improvements.3
Other upstream reproductive hormones may also impact bone.10 Indeed, recent in vitro and in vivo human data suggest that kisspeptin has positive effects on bone, potentially through kisspeptin receptors on osteoclasts and osteoblasts.11 Coupled with prior data indicating that kisspeptin can restore luteinising hormone pulsatility in FHA, this renders kisspeptin an exciting potential therapeutic for both bone and menstrual health in FHA.
CONCLUSIONS
FHA poses a significant risk to women’s bone health. Where required, current evidence supports transdermal HRT as the optimal form of oestrogen replacement, with superiority over alternatives. Continued research efforts are warranted to refine existing and identify new treatment strategies to optimise bone health in women with FHA.
JOVANNA TSOUTSOUKI
Section of Endocrinology and Investigative Medicine, Imperial College London
ALEXANDER N COMNINOS
Section of Endocrinology and Investigative Medicine, Imperial College London, and Endocrine Bone Unit, Department of Endocrinology, Imperial College Healthcare NHS Trust, London
REFERENCES
- Gordon CM et al. 2017 Journal of Clinical Endocrinology & Metabolism https://doi.org/10.1210/jc.2017-00131.
- Kandemir N et al. 2018 Journal of Clinical Endocrinology & Metabolism https://doi.org/10.1210/jc.2018-00338.
- Behary P & Comninos AN 2022 Frontiers in Endocrinology https://doi.org/10.3389/fendo.2022.923791.
- Ackerman KE et al. 2019 British Journal of Sports Medicine https://doi.org/10.1136/bjsports-2018-099723.
- Strokosch GR et al. 2006 Journal of Adolescent Health https://doi.org/10.1016/j.jadohealth.2006.09.010.
- Misra M et al. 2011 Journal of Bone & Mineral Research https://doi.org/10.1002/jbmr.447.
- Aalberg K et al. 2021 BMJ Open Sport & Exercise Medicine https://doi.org/10.1136/bmjsem-2021-001112.
- Singhal V et al. 2019 Journal of Clinical Endocrinology & Metabolism https://doi.org/10.1210/jc.2018-02143.
- Kam GYW et al. 2000 Journal of Clinical Endocrinology & Metabolism https://doi.org/10.1210/jcem.85.5.6527.
- Mills EG et al. 2021 Endocrine Reviews https://doi.org/10.1210/endrev/bnab015.
- Comninos AN et al. 2022 Journal of Clinical Endocrinology & Metabolism https://doi.org/10.1210/clinem/dgac117.






