Franics Ebling (eft and Jo Lewis (right) with Russell Foster (centre), whose lab has identified the retinal mechanisms by which mammals detect changes in day length (see The Endocrinologist issue 122, page 6)
After 30+ years as an academic researcher, Francis Ebling suggests that the best advice for surviving winter is to use the Christmas break or February half term to fly south for sun and warmth: ‘Mauritius and St Lucia are particularly agreeable but I’m looking forward to the day when I head down under to watch Joe Root lead a side to retain the Ashes!’ In contrast, as an early career researcher and impoverished parent, Jo Lewis’ advice adopts the more economical strategy of a thick sweater and warm coat. But for guidance of a more endocrinological nature, read on…
By the time you’re reading this, it will be too late to take action for winter 2016/17. In fact, most of our fellow species that are indigenous to Britain will be preparing for spring! Preparations for winter started soon after the summer solstice, as the gradual shortening of day lengths signalled that breeding should finish, fat stores be replenished, winter coat be grown, and a winter survival strategy enacted. For many of our avian friends, the winter plan is to head south of the equator to warmer climes where the day lengths are longer and food supply plentiful.
Our terrestrial counterparts obviously don’t have the option of a winter migration, but delving into a hibernaculum is perhaps an equally agreeable strategy. Actually, very few mammalian species indigenous to Britain truly hibernate, just a few species of bats and the dormouse – and when did you last see a dormouse, other than in the local panto production of ‘Alice in Wonderland’, played by someone evicted from the celebrity jungle?
Rather, many small mammals such as shrews and voles enter daily periods of torpor, a hypometabolic state where body temperature drops towards ambient air temperatures for part of each day, and heart rate decreases hugely. Torpor bouts usually occur during daylight hours, when small mammals would be largely inactive or sleeping at other times of the year.
UNDERLYING MECHANISMS
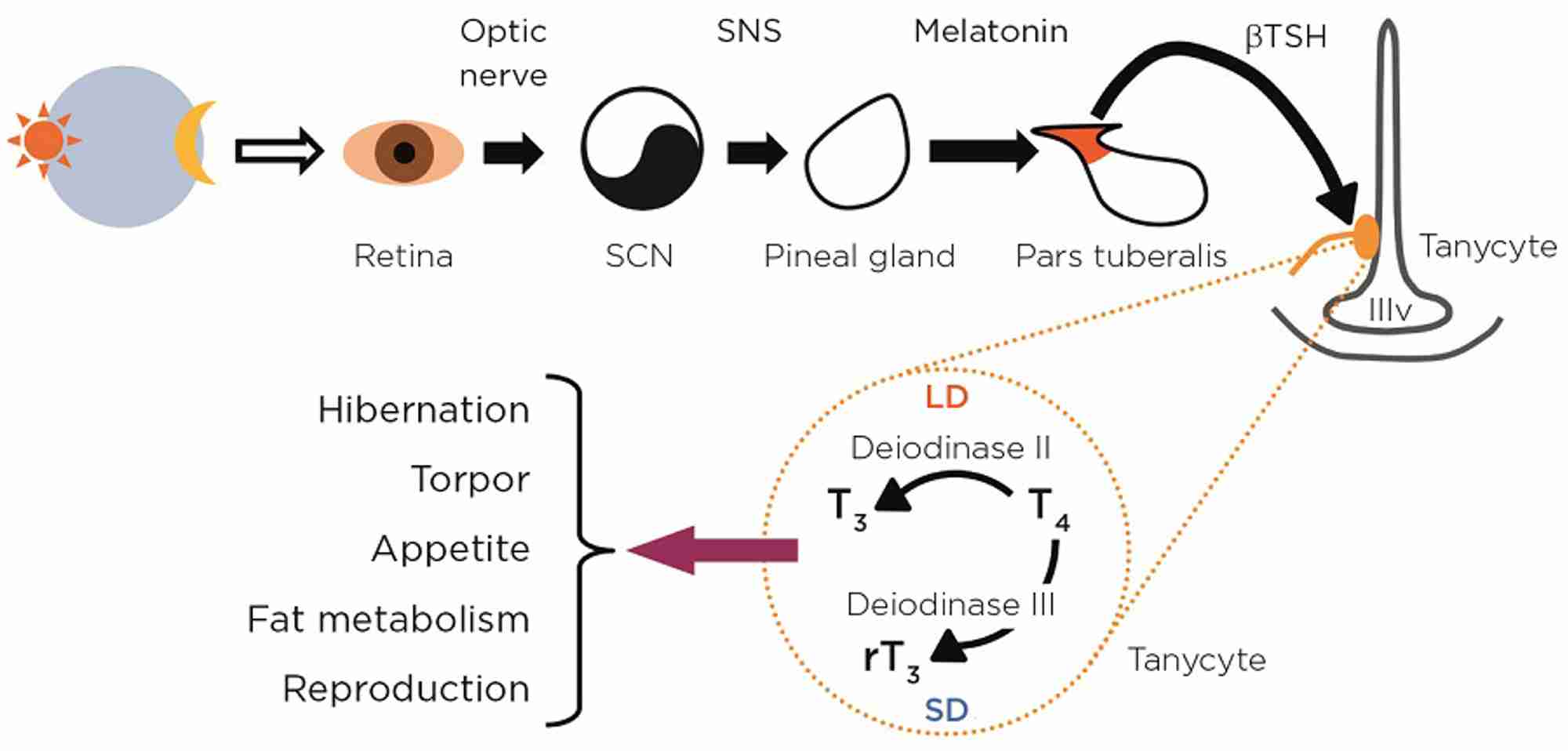
Our understanding of the neuroendocrine mechanisms that prepare mammals for winter stems from the use of ‘model’ seasonal species such as the Siberian hamster that can easily be maintained and studied under controlled laboratory conditions. The daily timing of torpor bouts clearly depends upon the suprachiasmatic nucleus in the hypothalamus (see Figure), as hamsters with lesions of this structure still show bouts of torpor when exposed to winter photoperiods, but they occur at random times of day. We also know that the increased duration of pineal melatonin secretion at night provides the crucial neurochemical signal encoding photoperiodic information in mammals.
More surprisingly, research in the last decade has revealed that the pars tuberalis in the pituitary stalk expresses a high density of melatonin receptors in all mammalian species, and is a key site of action of melatonin (Figure). Paracrine signals including the β-subunit of thyroid-stimulating hormone (βTSH) communicate from the pars tuberalis to the adjacent hypothalamus, targeting tanycytes. Another recent discovery is that tanycytes regulate hypothalamic availability of thyroid hormone through differential expression of deiodinase II and III genes.
Many of the winter adaptations in hamsters and other small mammals depend on a reduced availability of tri-iodothyronine (T3) in the hypothalamus. Experimentally, the induction of torpor, hypophagia and reproductive regression by short photoperiods can all be blocked by artificially maintaining high levels of thyroid hormone in the hypothalamus. Recent work from David Hazlerigg’s lab indicates that the regulation of seasonal neuroendocrine cycles by changes in hypothalamic thyroid hormone availability is an ancestral mechanism common across all vertebrates.
Whilst man is often considered to be a non-seasonal species, reflecting our evolutionary origins in equatorial Africa, there is increasing evidence of underlying seasonal traits in health and well-being. Might these also reflect changes in central thyroid hormone processing?
POTENTIAL TRANSLATIONAL BENEFITS
Understanding the neural and endocrine mechanisms underlying winter adaptations is fascinating biology in its own right, but has potential translational benefits in many areas.
'In preparing for winter, many mammals store huge amounts of fat ... Understanding how we can be healthily overweight might be a more tractable approach to managing the obesity epidemic'
First, in preparing for winter, many mammals become hyperphagic and store huge amounts of fat but, as far as we know, this is not associated with hepatic or muscular steatosis, or with insulin resistance, or with any other morbidities common in obesity. Understanding how we can be healthily overweight might be a more tractable approach to managing the obesity epidemic than proposing lifestyle or dietary changes that simply don’t work in the long term.
Secondly, many seasonal mammals then enter a prolonged period of programmed hypophagia coupled with increased fat oxidation. The adaptive value of this for surviving anticipated periods of reduced food availability is obvious, but if we can understand the central mechanisms underlying these traits will this identify novel therapeutic strategies to promote the same outcomes in man?
Thirdly, mammals that hibernate or show daily torpor retain normal brain function despite very hypoglycaemic and hypoxic conditions for prolonged periods. We already exploit hypothermia for some surgical procedures, but understanding the mechanistic basis for this remarkable neuroprotection should surely identify novel therapeutic targets to slow neurodegeneration and protect against ischaemic damage.
Francis J P Ebling (Professor of Neuroendocrinology) & Jo E Lewis (Research Fellow)
School of Life Sciences, University of Nottingham Medical School, Queen’s Medical Centre