Polycystic ovary syndrome (PCOS) is a very common endocrine disorder which causes both reproductive and metabolic dysfunction. The cardinal features are oligo- or anovulation (leading to menstrual irregularity and subfertility) and hyperandrogenism (associated with acne, hirsutism and alopecia). Metabolic dysfunction underlies problems with long term health, including increased prevalence of type 2 diabetes (T2DM), dyslipidaemia, fatty liver and higher risk of cardiovascular disease.
The manifestation of PCOS is typically heterogeneous, but there is strong evidence of a genetic basis for its aetiology. Recent genome-wide association studies (GWAS) have highlighted a shared genetic origin across the spectrum of clinical presentation.1,2 There is also evidence, principally from animal models of PCOS, that epigenetic programming by exposure to excess androgen during development has a role in its aetiology.3 But, as with other complex endocrine disorders, such as T2DM, there are important environmental factors, notably diet and lifestyle, that interact with genetic influences and may modify epigenetic effects.
'Resting energy expenditure was similar but, for equal degrees of obesity, women with PCOS were more insulin-resistant and had lower energy expenditure after a test meal than controls. Insulin resistance and PPT were negatively correlated in PCOS but not in controls'
OBESITY AND PCOS
Obesity in women with PCOS is associated with a greater degree of insulin resistance than in obese controls, and increasing body mass index (BMI) has a significant negative impact on reproductive and metabolic function, and long term health. Clinic-based studies typically report that most women with PCOS are obese, but a better indication of the prevalence of overweight and obesity in PCOS comes from population-based studies. Data from studies of the North Finland Birth Cohort (NFBC 1966) confirm a higher prevalence of obesity amongst women with symptoms of PCOS than in the reference population (26 vs 8% at age 31, increasing to 43 vs 22% at age 44).4 A similar trend, over 10 years, was seen in the Australian Longitudinal Study of Women’s Health.5,6
So, why the higher prevalence of obesity in PCOS? Does PCOS predispose to obesity or does obesity expose underlying PCOS? Both are probably involved, but there does appear to be a predisposition to obesity in women with PCOS. In the 2018 GWAS meta-analysis of women of European descent (the international PCOS genetic consortium), Mendelian randomisation analyses indicate that variants in genes associated with BMI play a causal role in PCOS.1 Low birth weight is a risk factor for later development of PCOS and, in the NFBC, adiposity rebound in childhood (a predictor of later obesity) was earlier in the PCOS cohort than in the reference population.7
ENERGY BALANCE AND PCOS
What do we know about energy balance in women with PCOS? There is no consensus about whether appetite and food intake are altered in PCOS. Eating disorders appear be more common in PCOS, but these are probably secondary to concerns about weight gain and the now well-described negative effect of PCOS on mental health. So, is energy expenditure altered in women with PCOS?
An important element of energy expenditure is postprandial thermogenesis (PPT) which accounts for about 15% of daily energy expenditure. We measured energy expenditure by indirect calorimetry in women with PCOS and control subjects.8 As reported in other studies,9 resting energy expenditure was similar but, for equal degrees of obesity (but higher abdominal fat), women with PCOS were more insulin-resistant and had lower energy expenditure after a test meal than (individually matched) controls. Insulin resistance and PPT were negatively correlated in PCOS but not in controls. It was estimated that, for the same calorie intake, women with PCOS would add 1.9kg more of fat over the course of a year, compared with those without PCOS. It is tempting to speculate that there is an evolutionary advantage in having reduced PPT. At times of food excess, reduced PPT in PCOS contributes to obesity, infertility and increased risk of diabetes. But, at times of food shortage, women without PCOS are more vulnerable to reduced fertility due to negative energy balance, whereas reduced PPT in PCOS protects energy expenditure and maintains fertility.
We considered the possible mechanisms underlying reduced PPT in PCOS, and first investigated whether it was driven by exposure to excess androgen during development. We studied a clinically realistic animal model of PCOS, the prenatally androgenised sheep, and found that the androgenised animals had a remarkably similar reduction in PPT to that seen in women with PCOS.10 There were associated changes in structure and function of adipose tissue, including reduced sympathetic drive and lower capacity for thermogenesis. Significantly, there was reduced central sympathetic nervous system activity, which was associated with decreased insulin signalling in the brain.
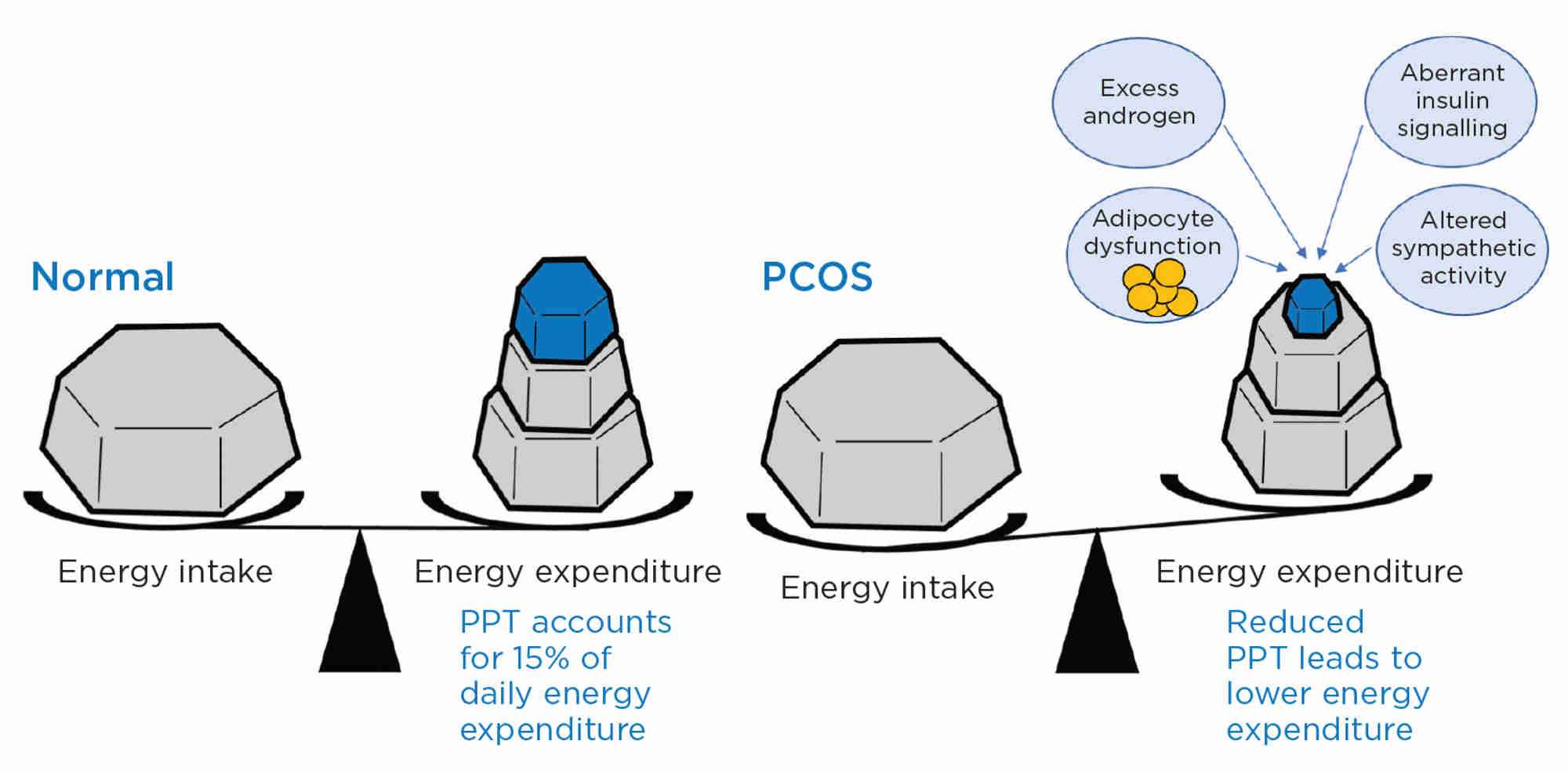
Figure. Post-prandial energy expenditure (thermogenesis; PPT) is reduced in women with PCOS and contributes to obesity. The probable mechanism(s) of reduced PPT involve(s) an interaction of several factors including excess androgen action, adipocyte dysfunction, aberrant insulin signalling (including in the brain) and altered sympathetic nervous system activity, both in fat and centrally.
MODIFYING ENERGY BALANCE IN WOMEN WITH PCOS
Of course, reducing calorie intake is a time-honoured method of improving energy balance and losing weight, but calorie-restricted diets are rarely sustainable. Can we instead or (more likely) in addition increase energy expenditure? Clearly increasing daily exercise has an important place in lifestyle modification, but is there a way of increasing PPT which, as suggested by the studies detailed above, would be particularly helpful in women with PCOS? Working on the premiss that insulin signalling in the brain is a key element in mediating diet-induced energy expenditure, the androgenised sheep were given intranasal insulin which significantly enhanced PPT. Are these remarkable findings translatable to the clinical realm? Clearly there is much more to be learned about energy balance in PCOS, but the results of these studies in the sheep with ‘PCOS’ have pointed the way to preliminary clinical studies.
Stephen Franks
Professor of Reproductive Endocrinology, Imperial College London
Colin Duncan
Professor of Reproductive Medicine and Science, University of Edinburgh
REFERENCES
- Day F et al. 2018 PLoS Genetics 14 e1007813.
- Dapas M & Dunaif A 2020 Current Opinion in Endocrine & Metabolic Research 12 26–32.
- Abbott DH et al. 2002 Journal of Endocrinology 174 1–5.
- Ollila MM et al. 2016 Journal of Clinical Endocrinology & Metabolism 101 739–747.
- Teede H et al. 2013 Obesity 21 1526–1532.
- Awoke MA et al. 2021 Human Reproduction 37 129–141.
- Koivuaho E et al. 2019 International Journal of Obesity 43 1370–1379.
- Robinson S et al. 1992 Clinical Endocrinology 36 537–543.
- Roumaldi D et al. 2019 Journal of Endocrinological Investigation 42 1089–1097.
- Siemienowicz K et al. 2020 iScience 23 101164.






